1. Municipal solid waste
Part I: Nature and amounts
Part I: Nature and amounts
1.1 Introduction
Municipal solid waste (MSW) is composed of household waste and trade and commercial waste and is so called because in ‘developed’ countries it is the responsibility of the municipality in which it is generated to remove it and dispose of it. Amounts of such waste are huge, and quantities in selected places are given in the table below. Comments follow the table.
Municipal solid waste (MSW) is composed of household waste and trade and commercial waste and is so called because in ‘developed’ countries it is the responsibility of the municipality in which it is generated to remove it and dispose of it. Amounts of such waste are huge, and quantities in selected places are given in the table below. Comments follow the table.
Place
|
Amount of MSW
|
Reference
|
London, Ontario.
|
267000 tonnes in 2006
|
[1] Asase M., Yanful E.K., Mensah M., Stanford J. and Amponsah S. ‘Comparison of municipal solid waste management systems in Canada and Ghana: A case study of the cities on London Ontario and Kumasi Ghana’ Waste Management 29 2779-2786 (2009)
|
Kumasi, Ghana.
|
365000 tonnes in 2006
|
[1] Asase M., Yanful E.K., Mensah M., Stanford J. and Amponsah S. ‘Comparison of municipal solid waste management systems in Canada and Ghana: A case study of the cities on London Ontario and Kumasi Ghana’ Waste Management 29 2779-2786 (2009)
|
China
|
180 million tonnes expected for 2010
| [2] Cheng H., Hu Y. ‘Municipal solid waste as a renewable source of energy: Current and future practices in China’ Bioresource Technology 101 3816-3824 (2010) |
UK
|
34.8 million tonnes in 2007/2008
|
[3]http://www.renewablefuelsagency.gov.uk/_db/_documents/Appendix_5_-_MSW_Case_Study.pdf
|
USA
|
190 million tonnes in 2009
|
[4] http://wihresourcegroup.wordpress.com/2009/05/13/solid-waste-management-consulting-firmsseek- out-china-for-business/
|
Australia
|
43.8 million tonnes in 2006/7
|
[5] http://www.abs.gov.au/AUSSTATS/abs@.nsf/Lookup/4613.0Chapter40Jan+2010
|
South West England
|
522 kg per resident in 2001
|
[6] http://www.steppingforward.org.uk/scen/msw.htm
|
Reference [1]([1] Asase M., Yanful E.K., Mensah M., Stanford J. and Amponsah S. ‘Comparison of municipal solid waste management systems in Canada and Ghana: A case study of the cities on London Ontario and Kumasi Ghana’ Waste Management 29 2779-2786 (2009)), from which the information in the first two rows of the table is taken, is a comparison of MSW production and management in two cities of widely differing ‘standards of living’: London ON and Kumasi Ghana, respective populations 0.35 millions and 1.61 millions. This gives a per capita daily production of 2.1 kg for London ON and 0.62 kg for Kumasi. The difference of a factor of three in amounts is accompanied by a difference in composition for MSW between London ON and Kumasi [1]. There is very much more paper in the London ON waste and more by way of waste from fruit and vegetables in the Kumasi waste. In London ON people often buy fruit and vegetables already peeled and processed. Such processing will not take place locally, London ON having no significant food industry. The waste will therefore go into the industrial or commercial waste stream at whatever places they are produced. In Kumasi by contrast fruit and vegetables will be purchased ‘straight from the land’ and the inedible parts will go into the domestic waste. On the other hand, the processed products in London ON will come in paper wrappings which find their way into the domestic waste stream there.
The third row gives the figure for the whole of China, population 1.4 billion; it translates to a per capita figure of 0.35 kg per day. That for the UK, population 61 millions, is in the next row, and this converts to a per capita figure of 1.6 kg per day. The trend observed above in comparison of cities – a higher per capita amount for the developed community – is shown for whole countries by this comparison of the UK with China. The figure in the next row is for the USA, the world’s largest producer of MSW by a fairly narrow margin over China. The per capita figure for the USA (population 315 millions) is 1.65 kg per day, remarkably close to that for the UK. The next row contains information for Australia, population 22.2 millions.
A point touched on earlier which will be developed later in the book is that variations in amounts and composition of MSW vary between places and cultures. Nevertheless, wherever people dwell and in whatever way MSW, or its equivalent in places not having a municipal structure, will be produced. The estimated population of the world in 2010 is 6.7 billion. On the basis of about 1 kg per person per day of MSW this becomes about ≈ 7 million tonne per day. MSW as formed has a low bulk density, perhaps 100 kg m-3, whereupon this figure becomes 70 million cubic metres per day.
Anticipating the next chapter on combustion of such waste and also the following section of this chapter in which calorific values will be discussed, the present author has shown previously [7] ([7] Jones J.C. ‘Selected examples of fuel use of waste and greenhouse implications’ Air, Water and Environment International December 2006 pp 14-18.) that a barrel of oil and a tonne of MSW release about the same amount of heat when burnt, approximately 7GJ. World consumption of oil is 80 million barrels per day. Not quite all of this goes into ‘combustion’ as some is diverted to petrochemical manufacture. Something like a tenth of the daily oil usage could according to this reasoning be replaced by MSW. In fact this simple calculation, though it gives an interesting perspective, does not extend to reality. There are many reasons why MSW is not equivalent in other ways to oil and, as the author put it in a recent talk (subsequently published as [8] ([8] Jones J.C. ‘Energy resources for the past, present and future’ Open Thermodynamics Journal – in press.)), nobody compos mentis would offer for a tonne of solid waste the price of a barrel of oil. Indeed, MSW might well have a negative financial value, that is, it might incur disposal charges. Even so MSW has over the decades found fuel use and there is much R&D into this at the present time. A factor in MSW handling by any means is composition variability and this will be discussed in the next section.
1.2 Composition
A study of the composition of MSW from a number of Asian countries [9] ([9] Hunsicker M.D., Crockett T.R., Labode B.M.A. ‘An overview of municipal waste incineration in Asia and the former Soviet Union’ Journal of Hazardous Materials 47 31-42 (1996)) and comparison with the waste from the USA revealed the following trends. MSW in India and China had a higher content of putrescible food waste than MSW in the US, respectively 36, 46 and 26%. This is of course the component of MSW which attracts microbial infection with accompanying hazards to community health. The MSW from China and India was found to be remarkably low in paper and cardboard, 5% and 3% in contrast with 41% for the USA and 37% for Japan. The proteins and fats in the food waste are of course combustible and contribute to the heat release in burning of the waste (although high moisture might make for delayed ignition) as are paper and cardboard. Paper and cardboard being cellulosic products have good calorific values (≈ 17 MJ kg-1) and are perhaps the most desirable constituent if burning of MSW is aimed for. There is also a marked difference in plastics contents between MSW from the countries considered in [9]: Japan 15%, Taiwan 21%, China 1%, India negligible and the USA 6%. Plastics are also helpful in eventual combustion. Some have calorific values of around 40 MJ kg-1, approaching the values for petroleum products. There is however one difficulty: the burning of PVC results in formation of dioxin, the most harmful substance to humans known. Atmospheric levels of pg m-3 apply, and sudden release of a quantity of 1 kg is a major incident. Monitoring for dioxin in post-combustion gases is possible, and is required when PVC waste is being destroyed by burning. There will also be some textile waste in MSW to the extent of up to about 4% and some wood (e.g., from tooth picks). There will also of course be glass and metals in MSW, perhaps between 5 and 10%. These might have some value, but their importance to the topic of thermal treatment of wastes is that they neither burn nor pyrolyse.
A study of the composition of MSW from a number of Asian countries [9] ([9] Hunsicker M.D., Crockett T.R., Labode B.M.A. ‘An overview of municipal waste incineration in Asia and the former Soviet Union’ Journal of Hazardous Materials 47 31-42 (1996)) and comparison with the waste from the USA revealed the following trends. MSW in India and China had a higher content of putrescible food waste than MSW in the US, respectively 36, 46 and 26%. This is of course the component of MSW which attracts microbial infection with accompanying hazards to community health. The MSW from China and India was found to be remarkably low in paper and cardboard, 5% and 3% in contrast with 41% for the USA and 37% for Japan. The proteins and fats in the food waste are of course combustible and contribute to the heat release in burning of the waste (although high moisture might make for delayed ignition) as are paper and cardboard. Paper and cardboard being cellulosic products have good calorific values (≈ 17 MJ kg-1) and are perhaps the most desirable constituent if burning of MSW is aimed for. There is also a marked difference in plastics contents between MSW from the countries considered in [9]: Japan 15%, Taiwan 21%, China 1%, India negligible and the USA 6%. Plastics are also helpful in eventual combustion. Some have calorific values of around 40 MJ kg-1, approaching the values for petroleum products. There is however one difficulty: the burning of PVC results in formation of dioxin, the most harmful substance to humans known. Atmospheric levels of pg m-3 apply, and sudden release of a quantity of 1 kg is a major incident. Monitoring for dioxin in post-combustion gases is possible, and is required when PVC waste is being destroyed by burning. There will also be some textile waste in MSW to the extent of up to about 4% and some wood (e.g., from tooth picks). There will also of course be glass and metals in MSW, perhaps between 5 and 10%. These might have some value, but their importance to the topic of thermal treatment of wastes is that they neither burn nor pyrolyse.
1.3 Calorific values
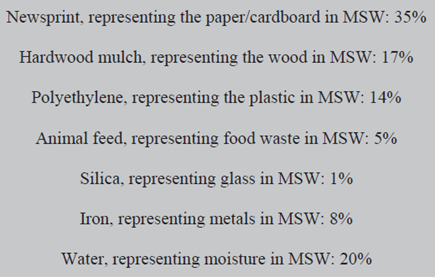
This was touched on in the previous section, where a comparison with crude oil was made, and will be more quantitatively examined in this section. It was because of the variability of composition of MSW even from a particular place that an investigation into the calorific value of MSW from the USA [10] ([10] Thispe S.S., Sheng C., Booty M.R., Magee R.S., Bozzelle J.W. ‘Chemical makeup and physical evaluation of a synthetic fuel and methods of heat content evaluation for studies of MSW incineration’ Fuel 81 211-217 (2002)) used simulated MSW, made from controlled amounts of well characterised components blended in such proportions as to represent MSW of typical composition. The composition of the simulated waste in [10] is summarised the in shaded area below.
This was touched on in the previous section, where a comparison with crude oil was made, and will be more quantitatively examined in this section. It was because of the variability of composition of MSW even from a particular place that an investigation into the calorific value of MSW from the USA [10] ([10] Thispe S.S., Sheng C., Booty M.R., Magee R.S., Bozzelle J.W. ‘Chemical makeup and physical evaluation of a synthetic fuel and methods of heat content evaluation for studies of MSW incineration’ Fuel 81 211-217 (2002)) used simulated MSW, made from controlled amounts of well characterised components blended in such proportions as to represent MSW of typical composition. The composition of the simulated waste in [10] is summarised the in shaded area below.
It ought to be easy enough to estimate the calorific value (CV) of this to within 10% or so. This is in the boxed area below.
In [10] this is compared with values recorded at a MSW facility in Delaware, which range from 8.4 to 17.6 MJ kg-1 with an average of 11.3 MJ kg-1. The value for the simulated waste [10] just exceeds the upper limit of that range, and the reason is that the water content of 20% is low. Whilst MSW can be as low in water as that about 50% would be more typical and 70% not impossible.
In the table below some more literature values are given. Comments follow the table.
Origin of the MSW
|
Water content %
| Calorific value/MJ kg-1 |
Reference
|
Changzhou, China
|
48.5
|
3.0
|
[11] Zhang Y., Chen Y., Meng A., Li Q., Cheng H. ‘Experimental and thermodynamic investigation of transfer of cadmium influenced by sulphur and chlorine during MSW incineration’ Journal of Hazardous Materials 153 309-319 (2008)
|
Guangzhou, China
|
50.1
|
4.4
|
[11]
|
| Kuala Lumpur, Malaysia |
55.0
| In the range 10.0 to 16.8 |
[11]
|
Parona, Italy
|
20 to 30
| In the range 10.5 to 16.7 |
[11]
|
Paris
|
35
|
8.4
|
[13] Desroches-Ducarne E., Marty E., Martin G., Delfosse L. ‘Co-combustion of coal and municipal solid waste in a circulating fluidised bed’ Fuel 77 1311-1315 (1998)
|
Tehran
|
65
|
5.0
|
[14] Chokouhmand H. ‘Energy recovery from incineration of Tehran MSW and its air pollution effects’ Energy Conversion Management 22 231-234 (1982)
|
The high value for KL is due to large amounts of putrescible food waste (51.9%) and plastics (21.0%). A reader should compare these with the values for the respective constituents given in the shaded area above. A similar value for KL from a totally independent investigation is given in [12]([12] Kathiravale S., Yunus M.N.M., Sopian K., Samsuddin A.H., Rahman R.A. ‘Modelling the heating value of municipal waste’ Fuel 82 1119-1125 (2003)). Reference [14], which long predates any of the other references in the table, also gives figures for a number of South East Asian cities. For example, the figure for Hong Kong at 60% moisture is given as 9.3 MJ kg-1 which is broadly consistent with the much more recent figure in the table for KL.
When the calorific value of a fuel is determined in a bomb calorimeter the value obtained is the higher heating value (HHV). This is the value on the basis that all of the product water condenses and in so doing contributes the heat effect of the phase change to the calorific value. This is in contrast to the lower heating value (LHV), which is the heat obtained if the water in the products remains in the vapour phase. Someone examining the literature for calorific values might not always be informed expressly whether the value given is the HHV or the LLV. The calculation in the boxed area below addresses this point.
From [12], the hydrogen content of dry MSW from KL is 6.86%. The MSW is however burnt at a moisture content of 55%. One kilogram of the MSW as fired therefore contains:
(68.6 × 0.45) g hydrogen = 31 g hydrogen or 15.5 mol (expressed as H2)
↓
15.5 mol of water on combustion
Using a value of 44 kJ mol-1 for the heat of vaporisation of water at 25°C, the heat released on the condensation of 15.5 mol of water at that temperature =
44 × 103 × 15.5 × 10-6 MJ = 0.7 MJ
So for a calorific value in the range 10 to 15 MJ kg-1 the HHV and LHV differ by something like 5%
|
It is repeated that if the determination of the calorific value was in a bomb calorimeter it is CERTAIN that it corresponds to the HHV. If such information is not given an uncertainty of about 5% results. This is comparable to errors in the determination of the calorific value of MSW in a bomb calorimeter [10]. Errors would not be this large in the laboratory measurement of the HHV of a coal, where much more uniform samples for the calorimetric work can be obtained than can for MSW.
1.4 Constituents of MSW other than household waste
Local authorities will collect, in addition to waste from households, waste from some commercial premises. At present about 10% of the MSW generated in the UK is of trade rather than domestic origin [15] ([15] Burnley S.J. ‘A review of municipal solid waste composition in the UK’ Waste Management 27 1274-1285 (2007)). Any waste incorporated into the MSW must be of comparable composition to household waste and must have no hazards additional to those of household waste. Obviously therefore, hospital waste would not be incorporated into MSW. It is reported in [15] that ‘commercial waste’ collected in London (UK) is as high as 64% in paper and cardboard and about 11% in plastics including plastic film used in wrapping. Litter from bins mounted in the street also finds its way into MSW, and debris from fast food outlets features strongly in this.
1.5 Carbon neutrality or otherwise of MSW as a fuel
Clearly some constituents of MSW are carbon-neutral and others are not. In the former category are paper and vegetable peel and in the latter are plastics. Commonly MSW is taken to be 50 to 70% carbon neutral. Because plastics have about twice the calorific value of cellulose their contribution to their heat release on combustion is disproportionate to their weight contents, a factor requiring thought when carbon dioxide emissions are being calculated.
1.6 Trade wastes
‘Waste from commercial premises’, discussed in the previous section, is distinct from ‘trade waste’. Trades and industries produce solid waste peculiar to their particular activities and processes. Such waste might, like MSW, be suitable for fuel use. Details of a few such wastes are given in the table below. Comments follow the table.
Trade or industry
|
Amounts of waste and calorific value
|
Furniture
|
1 million tonnes of lignocellulosic waste from furniture manufacture per year in the UK [16]([16] http://www.trada.co.uk/techinfo/research/EnviroFibre.htm). Calorific value ≈ 17 MJ kg-1
|
Vehicle tyres
|
Tens of millions of tyres scrapped in the UK each year.
Calorific value ≈ 30 MJ kg-1 [17]([17]http://www.appletonlemoors.co.uk/docs/calorific_values.PDF) |
Leather
|
20 million tonnes of leather waste per year in the UK
Calorific value ≈ 20 MJ kg-1 |
Citrus fruit products
|
5 million tonnes per year of orange peel produced in the US [18]([18] http://asae.frymulti.com/abstract.asp?aid=21005&t=2). Calorific value ≈ 5 MJ kg-1
|
Offices
|
80 million tonnes of waste paper per year in the UK [19]([19] http://www.guardian.co.uk/environment/2008/feb/21/waste.recycling).
|
Wood waste such as that described in row 1 is a good fuel, being of calorific value about 17 MJ kg-1 and, perhaps more importantly, of much more consistent composition than MSW and not as unappealing to work with. Combustion is not necessarily the destiny of such waste, however, as there are products obtainable from it including fibre board. Combustion of scrap tyres has proved difficult over the years, the reason being that the latex from which they are made releases copious amounts of volatiles on initial heating and this makes for a smoky burn. However, there is a revival of interest because of the carbon neutrality of latex, in particular of co-firing of shredded tyre waste with coal in power generation. Citrus peel as a fuel has a strength and a weakness. The strength is that it is consistent in composition and in burning this makes for flame stability. The weakness is its low calorific value, due to the high moisture content. Sometimes a fuel is assessed on the number of times its own weight of saturated steam at one bar which it can raise, and one expects a value of not less than five for a coal obtained for steam raising. Citrus peel can only raise just over its own weight of saturated steam at one bar. A further disadvantage is that where there are large amounts of moisture in a fuel it is simply present before, during and after combustion. This means that large boiler furnace volumes are required to contain the vapour additional to the reactant and product gases.
Paper waste is ubiquitous and is of course a significant part both of MSW and of commercial waste. The figure in the fifth row of the table is for paper waste generated in offices. It provides a rationale for the rubric that sometimes accompanies an e-mail message that the contents should not be printed off unnecessarily.
1.7 Concluding remarks
The introductory chapter has given an account of the nature of MSW as a lead-in to subsequent chapters where burning, gasification and pyrolysis of MSW are described. The burning of MSW not merely to dispose of it but also to obtain some return on the heat is by no means new; the first such operation was at the NYC incinerator in the late nineteenth century. The scale of MSW production was pointed out, with emphasis, earlier in the chapter. Because of that and because MSW is partially carbon-neutral R&D into its fuel use is on-going.
next Municipal solid waste: Incineration
Hi Mr. Barua,
ReplyDeleteI apprecaite your work.
Why don't you send me an article based on F&B industry's solid waste management. It will be very useful to our industry readers.
PK
PROCESS India
prasanta.chatterjee@vogel.de
Cell: 91 9320912419