previous Municipal solid waste: Incineration
3. Municipal Solid Waste
Part III: Pelletised forms
3.1 Introduction

3. Municipal Solid Waste
Part III: Pelletised forms
3.1 Introduction
Refuse-derived fuel (RDF) is as its name informs one waste substance destined for fuel use. Possible treatments of waste to make RDF are many and include Mechanical Biological Treatment which has brief coverage later in this chapter. This chapter is concerned with pelletising of MSW to make what approximates to a general-purpose solid fuel. Processes involved in the manufacture of such pellets include drying, shredding and ‘densification’. Over the decades there has been much endeavour in making RDF pellets but it has only ever been on a modest scale, RDF never having seriously challenged coal or wood. There is increased interest at the present time, partly because of the partial carbon neutrality of such fuels.
As we saw in an earlier chapter MSW has a very reasonable calorific value, more so if it is dried to make RDF. One difficulty with RDF is that heterogeneity of composition of MSW makes for variation of composition. Another is that RDF pellets tend not only to be high in ash but that such ash is often corrosive to combustion plant. Another is that the MSW as received for processing might well contain pathogenic bacteria: that was largely the motive for getting it out of households in the first place! These issues will be raised again when particular examples of RDF pellets are described.
3.2 Manufacture of RDF pellets
3.2.1 Principles
A good summary of what is involved in making pelletised RDF from MSW is given in [1]([1] http://www.gec.jp/WASTE/data/waste_C-5.html). The information relates to an RDF manufacturing plant designed and built by the Japanese concern Kawasaki.MSW as received is crushed and blast dried, that is, treated with air previously electrically heated. This also brings about deodorisation of the MSW. Metals, glass and any other non-combustibles are then removed and this is followed by shredding in readiness for pelletisation, a.k.a. the ‘solidifying step’. This process is also referred to as ‘densification’ as noted in the previous section. In a suitable climate, the blast drying step can be replaced by solar drying.
Presses for making RDF pellets ‘evolved’ from those designed for making animal feed in the form of pellets [2]([2] http://pubs.acs.org/doi/abs/10.1021/bk-1980-0130.ch010). It is described in [3]([3] http://www.aseanenvironment.info/Abstract/41015154.pdf) how in the production of RDF pellets of cylindrical shape and of 15 mm diameter a force of 50 kN was applied axially. It is easily shown that the pressure experienced by the pellets during processing would have been:
50 × 103 N/[ π(7.5 × 10-3)2] m2 = 280 MPa
which is about half the design stress of a typical stainless steel [4]([4] http://www.bssa.org.uk/topics.php?article=125). RDF pellets will usually require a binder. In contrast to coal briquetting technologies which use an organic substance – either petroleum residue or a starch – as a binder, RDF pellet manufacture often uses an inorganic binder. Calcium hydroxide is a common choice. Where the waste from which the RDF was made contained large amounts of PVC a further inorganic additive might be used to fix the chlorine as a metal chloride in the ash on combustion, preventing its release as hydrogen chloride into the atmosphere. Magnesium hydroxide can be used as such an additive.
RDF is expected to have a calorific value of the order of 12 to 15 MJ kg-1. It is sometimes possible to raise the calorific value of RDF pellets by blending, prior to application of pressure, with a suitable trade waste such as carpet waste. Peanut shells and rice husk have also found such application. The term c-RDF, where ‘c’ stands for composite, is used to describe such fuels. Approximately synonymous is REF, ‘in-origin recycled fuel’.
RDF pellets might be used as the sole fuel for a particular plant or, increasingly frequently, co-fired with a conventional fuel. Details of combustion of RDF, with examples, will be discussed starting with the table below.
3.2.2 Selected scenes of RDF manufacture
Location
|
Details
|
Ref.
|
| Andhra Pradesh, India |
RDF pellets as fuel for electricity generation. Calorific value 12 to 13 MJ kg-1
Ash content 20% |
[5] http://www.3rkh.net/3rkh/files/RDF2power.pdf
|
| Herhof plant, Dresden, Germany |
Pre-treatment by ‘aerobic digestion’ before pelletisation (see below). Pellets of calorific value 15 to 18 MJ kg-1.
|
[7] http://www.aseanenvironment.info/Abstract/41014407.pdf
|
| Kahada-Okuise RDF plant, Japan. |
Calcium hydroxide binder used. Pellets of calorific value 18 to 20 MJ kg-1
|
[7] http://www.aseanenvironment.info/Abstract/41014407.pdf
|
| Istanbul, Turkey |
Pilot study into pelletised RDF production.
|
[8] Kara M., Gunay E., Tabak Y., Yildiz S. ‘Perspectives for pilot scale study of RDF in Istanbul’ Waste Management 29 2976-2982 (2009)
|
| Greve in Chianti, Italy |
RDF pellets of calorific value 17 MJ kg-1.
|
[9] http://www.tps.se/img/2008/1/30/15707.pdf
|
| Stockholm, Sweden |
CHP from fuels including REF pellets.
|
[10] http://www.fwc.com/publications/tech_papers/files/TP_CFB_08_02.pdf
|
Very interestingly, reference [5]([5] http://www.3rkh.net/3rkh/files/RDF2power.pdf) gives a value for the energy-return on energy invested (EROEI) for the RDF of 10 to 15. According to recent thermodynamic theories of energy-return-on-energy-invested for conventional fuels [6], this EROEI would apply to crude oil obtained from a well having a depth of about 2000 m. The fact that RDF is made from MSW which has to be disposed of would have the effect of raising the EROEI. This is because whatever energy would have been involved in taking the waste to a landfill instead of processing it to RDF can be subtracted from the ‘energy invested’.
At the Herhof plant described in row two of the table, following removal of non-combustibles there is treatment in air at 60°C for a week in a process called aerobic digestion. This is in effect natural composting accelerated by temperature. At the Herhof plant the material after aerobic digestion has a fluffy nature and it is this which is pelletised. In some applications the fluff is used as a fuel as obtained without pelletising. Comparing the calorific values of the pellets in rows two and three of the table, the indication is that the aerobic digestion at the Herhof plant has had a marginally unfavourable effect on the calorific value. If this is so (and much more evidence would be needed for the ‘indication’ to become even a tentative ‘conclusion’) it is not difficult to explain. The prolonged treatment at 60°C would have involved loss of low-temperature volatiles such as methanol and formaldehyde which, had they been devolatilised in burning instead of in pre-treatment, would have enhanced the calorific value.
In the pilot study in Turkey described in the next row, the moisture content of the pellets was 25%, not unusually high for such a fuel but too high for the intended use of the pellets. The difficulty with high moisture is not its effect on the flame temperature (although there certainly is such an effect) but the fact that evaporated water adds to the space required in a furnace (as noted in a previous chapter) and in flue gas removal. This makes for difficulties if, as is likely to be the case, the RDF pellets are to be used in plant previously taking a conventional fuel. It was mentioned in Chapter 1 that MSW, in raw or in pelletised form, is not necessarily destined for burning but can be gasified, to make a fuel gas which is itself burnt. Again a ‘sneak preview’ of a later section of the book is necessary as the gasification of waste is a wide topic requiring in a text such as this major treatment. That being said we note two points at this stage. First, a significant proportion of the RDF pellets at Greve in Chianti (row five of the table) are gasified to make a fuel gas. Secondly, whatever the effects on the EROEI of the conversion to gas such a gas has many advantages over RDF pellets including the obvious one of its giving a cleaner burn. The point about the excessive gas volume caused by water inherent in the fuel is noted in [10], which is concerned with a CHP plant in Sweden which draws on a miscellany of fuels according to price and availability. These include wood waste from demolition.
3.2.3 Carbon neutrality issues
Although pelletisation of MSW to make pelletised RDF is not new, much research activity into it in the last few years has found its way into peer-reviewed journals. The motivation for the work has been the stretching of conventional fuels, and two factors have necessitated this. One is that many countries have either reduced their coal production (e.g., the UK) or ceased coal production altogether (e.g. Japan). As already noted Japan relies on imports from countries including Australia and Indonesia and the UK produces of the order of 20 million tonnes per annum for the domestic market. A century ago she was producing about five times this. A renaissance of coal production and utilisation is by no means off the agenda, but if it occurs it will not be a simple ‘return to the past’. Disused mines cannot be brought back into production at a moment’s notice, and increased stringency of safety standards since coal production ceased in the UK will make for expensive infrastructure if mines are reopened. Also, the future for coal is not its burning as such but its gasification in what is sometimes called ‘BTU conversion’. The second factor having stimulated recent research into RDF combustion has been touched on already in this book: its partial carbon neutrality. Another point mentioned earlier is that RDF-coal co-firing is expanding and enabling such organisations as electricity producers to meet renewables obligations.
It is necessary to expand upon the matter of the carbon neutrality if we are knowledgeably to examine recent work on coal-RDF coal firing. What is required to meet carbon dioxide reduction requirements is not necessarily a reduction in total carbon dioxide release but a reduction in fossil fuel derived carbon dioxide release. Carbon dioxide released on the burning of a carbon-neutral substance was in the fairly recent past carbon dioxide in the atmosphere, so to burn a carbon-neutral fuel is to put carbon dioxide back where it came from. Having regard to the uptake of carbon dioxide by vegetation, return to the atmosphere of carbon dioxide from carbon-neutral fuels causes no net increase in the CO2 level. By contrast, carbon in coal was not on any time scale of interest carbon dioxide in the atmosphere, so to burn coal adds to the CO2 level of the atmosphere. The present author has published elsewhere (e.g. [11]([11] Jones J.C. ‘Selected examples of fuel use of waste and greenhouse implications’ Air, Water and Environment International December 2006 pp 14-18.), [12]([12] Jones J.C. ‘Reflections on combustion principles as they relate to a miscellany of practical fuels’ Chemical Journal of Armenia 60 (2) 174-185 (2007))) calculations which show that when in a combustion process a carbon-neutral fuel such as wood waste is fully or partially substituted for a bituminous coal the result, other things being equal, will be an increase in the total carbon dioxide release. The important difference is that carbon dioxide resulting from the carbon-neutral fuel, unlike that resulting from coal, makes no net contribution to the CO2 level of the atmosphere as explained above.
3.3 Performance issues
3.3.1 Preamble
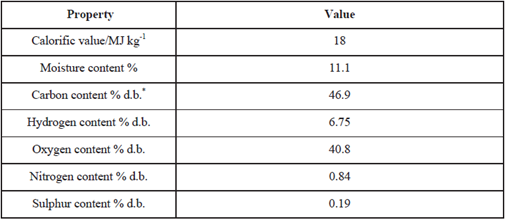
This section will take analysis and other data for representative RDF pellets and use them in calculations relevant to performance. The example of pelletised RDF used is taken from reference [13]([13] Piao G., Aono S., Mori S., Deguchi S., Fujima Y., Kondoh M.,Yamaguchi M. ‘Combustion of refuse-derived fuel in a fluidised bed’ Waste Management 18 509-512 (1998)). It originates in Nagoya, Japan, and information on it taken directly from [13] is given in the table below. It is clear that this RDF is one of fairly low moisture and correspondingly good calorific value and is a most suitable choice to represent RDF pellets generically in the calculations which follow.
3.3.2 Air requirement on burning
This is calculated in the shaded are below.
The above follows the procedure for fairly routine ‘combustion calculations’ for coal and oil, extending the ideas to RDF pellets. A reader should note the following.
- The factor of 3.76 by which the molar oxygen requirement is multiplied is the quotient 79/21, in which the numerator and denominator are the percentages molar basis respectively of nitrogen and oxygen in air.
- Oxygen in the fuel before burning signifies fuel already oxidised, so it has to be subtracted from the oxygen requirement. The sulphur in the RDF will go quantitatively to sulphur dioxide, a point to which we shall return when discussing emissions. The oxygen requirement for this is however so low that it can be neglected in the above calculations.
- Excess air to a degree of about 30% would be common in such an application.
The conclusion then is that a kilogram of the RDF pellets would require six kilograms of air for combustion. In addition to having calculated this result we can claim to have done at least a partial mass balance on the process. This we continue in the calculation of the composition of the flue gas.
3.3.3 Composition of the flue gas
Calculation of flue gas composition is in the shaded area below and begins with information from the previous calculation.
Once the gas had cooled say to 25°C the water would cease to be in the vapour phase and the total number of moles would be 263 per kg of the RDF pellets burnt. Now at 25°C and 1 bar pressure:
1 m3 of any gas or gas mixture contains approximately 40 mol
therefore the volume of gas produced in the burning of 1 kg of the pellets is:
263/40 = 6.6 m3
It is hoped that a reader might use these figures in order to enlarge upon those given for particular RDF facilities in the table previously presented. The calculations are extended below to the adiabatic flame temperature.
3.3.4 Adiabatic flame temperatures
The adiabatic flame temperature is the temperature attained when all of the heat released is retained as enthalpy (sensible heat) in the reaction products. It is an upper bound on actual realisable flame temperatures. The adiabatic flame temperature for the RDF pellets under consideration is calculated in the shaded area below. It first has to be pointed out that the adiabatic flame temperature is usually calculated for stoichiometric conditions, that is no excess air. The calculation below is for such conditions.
The heat capacities in the calculation are for a single temperature. A more rigorous treatment would incorporate the heat capacities as a function of temperature. That is probably the principal source of error in the above calculation which nevertheless has given about the value expected. We note [14] that the adiabatic flame temperature of methane in air under stoichiometric conditions is 2222°C (2495K). Very close comparison would not be helpful since the calculation herein for RDF is an approximate one. There is also a source of error in the use of a single value for the calorific value from [13] when this in fact will have a significant plus-or-minus on it. Even so the following conclusion can be drawn: in terms of combustion temperatures reached RDF pellets can hold their own against conventional hydrocarbon fuels.
3.3.5 Emissions
We first note that it is in general correct to equate the moles of elemental sulphur in the fuel to the moles of sulphur dioxide produced. In any fuel there is a stoichiometric conversion to sulphur to sulphur dioxide on burning even if conditions are fuel-rich. The one exception which is often cited is that in certain coals sulphur dioxide once formed can be further oxidised and become sulphates in the ash by combination with calcium or sodium. Having regard to the fact that the RDF in [13] does contain calcium amongst its ‘inorganics’ such behaviour is possible here. It would be straightforward to calculate how much sulphur at most could be fixed in this way from the calcium content, the rest becoming sulphur dioxide.
The sulphur dioxide concentration of 230 p.p.m. would need to be reduced, by scrubbing of the flue gas or by use of lime, by the factor estimated below.
The above result does not make for difficulties in operation. Plenty of coals are as high in sulphur as the RDF pellets in [13] as are some heavy fuel oils.
Whereas sulphur is quantitatively converted to sulphur dioxide on combustion, fuel nitrogen is converted quantitatively to nitrogen gas N2. A very small proportion which might hardly reveal itself in a routine mass balance calculation will go to NO and NO2, jointly referred to as NOx. This is called fuel NOx and contrasts with thermal NOx which is due to reaction of nitrogen and oxygen in the air. Thermal NOx occurs only at combustion temperatures of about 1300°C or higher. The role of NOx in atmospheric pollution has been described by the author elsewhere [15]([15] Jones J.C. ‘Atmospheric Pollution’ Ventus Publishing, Frederiksberg (2008), accessible on BookBoon.). NOx release into the atmosphere has to be controlled and regulated, and this applies to RDF and ‘conventional’ fuels alike.
Where RDF contains major amounts of chlorine, as it will if the MSW from which it is made contains PVC, calcium can be incorporated to trap it as calcium chloride preventing its release as HCl. An example of this is discussed in section 3.4.
3.4 Coal RDF co-firing
The table below summarises three recent activities in RDF pellet-coal co-firing, both investigative studies and plant which is ‘up and running’. Comments follow the table.
Reference
|
Details
|
[16] Wan H-P., Chang Y-H., Chien W-C., Lee H-T., Huang C.C. ‘Emissions during co-firing of RDF-5 with bituminous coal, paper sludge and waste tyres in a commercial circulating fluidised bed cogeneration boiler’ Fuel 87 761-767 (2008)
|
RDF pellets of calorific value 24 MJ kg-1 made from paper and plastic waste co-fired in a fluidised bed with a bituminous coal of calorific value 21 MJ kg-1, also paper sludge and tyre-derived fuel. Five large-scale tests performed each of one week’s duration.
|
[17] http://www.art-environmental.com/downloads/eu-thermie.pdf
|
Slough, England. Coal and pelletised waste co-fired in a fluidised bed for electricity generation. Heat contribution 40% from the waste 60% from the coal.
|
[18] Chyang C-J., Han Y-L, Wua L.W., Wan H-P, Lee H-T , Chang Y-H. ‘An investigation on pollutant emissions from co-firing of RDF and coal’ Waste Management – in press.
|
RDF pellets and coal co-fired in a fluidised bed reactor. Inclusion of CaCO3 to trap chlorine.
|
In interpreting the results in reference [16] we first note that a ‘cocktail’ of three waste-derived fuels and one conventional one was used. The very high calorific value of the RDF pellets is due to their high plastics content and their low moisture content. On burning of this sulphur dioxide levels of about 200 p.p.m. in the flue gases were observed. The bed operated at about 900°C, too low for there to be thermal NOx. Measured NOx levels of up to 80 p.p.m. in the flue gas were therefore fuel NOx entirely.
At the plant at Slough which features in the second row of the table, some of the waste fuel is RDF pellets and some consists of small cubes – typically 3 cm side – of compressed cellulosic waste. The advantage of cellulosic waste is that it is entirely carbon-neutral whereas RDF from MSW is only partially so. Accordingly electricity from the plant is sold to electricity producers to enable them to meet their nonfossil fuels obligations. Sulphur dioxide produced at the Slough facility is removed by inclusion of limestone in the fluidised bed. The bed temperature is too low for thermal NOx to be formed.
When calcium carbonate is being use to trap chlorine as in the study described in the third row of the table, the efficacy of the trapping can be assessed in the following way. The amount of chlorine in the waste-derived fuel is measured and, in experimental trials, amounts of calcium (as carbonate as noted) in various multiples in molar terms of the chlorine are injected into the combustion system. The ash can be analysed for chlorine, and that expressed as a function of the molar ratio of calcium to chlorine (Ca:Cl). The higher the chlorine level in the ash the more effective the calcium has been in removing it from the gas phase. In [18], the chlorine content of the ash was 0.1% when there was no calcium carbonate injection at all, rising to ≈ 0.14% for Ca:Cl = 5, to ≈ 0.2 for Ca:Cl = 10 and to ≈ 0.25 for Ca:Cl = 15. A large excess of the calcium carbonate is therefore needed for a good result.
We observe from several of the examples of waste-derived fuels examined so far in this book that fluidised beds are often preferred over, for example, grate combustion in the burning of wastes. Fluidised beds are often used for poorer fuels. The value of the fluidised bed has been explained to countless students at Aberdeen by the following analogy. If an electric iron is set at too high a temperature for the fabric to which it is to be applied it will create a hole in it. However, if air at the same temperature as the iron is directed at the fabric it is much less likely that damage will result. With the hot iron heat transfer is by conduction: with the hot air it is by convection. In the latter case the fabric will never get to the air temperature because of heat transfer from itself to the surroundings leading to an equilibrium temperature well below that of the air. In a fluidised bed heat to the fuel particles is received by conduction from the fluidised material which will consist of inert particles, often sand. This makes for a rapid heating rate of the fuel particles to the acceleration of combustion.
3.5 Concluding remarks
It has never happened on a wide scale that RDF pellets have become a general-purpose solid fuel for distribution as, for example, coal briquettes have. Where we are seeing major activity into RDF pellets is in electricity generation where three factors are in their favour: their low cost in comparison with oil and coal, their partial carbon neutrality and the advantages of RDF production over landfill disposal of MSW. So will producers of RDF pellets ever be, on a large scale, stockpiling before transportation of the pellets by road and rail to users? The need for safe storage practices with large volumes has been recognised in a program of research in Japan into self-heating in stockpiles [19]. Additional to self-heating is the possibility of hydrogen production from RDF by micro-organisms.
The idea that RDF pellets might be exported from one country to another cannot be dismissed, as raw MSW not even destined for fuel use is sometimes transferred between countries. A good deal of the waste which goes to landfills in the US state of Michigan is imported there from the Canadian Province of Ontario. Payment for that is from Canada to the US so it can be described as a ‘negative export’ from Canada. That RDF pellets should ever become a major ‘positive export’ is at first consideration unlikely in that no country is short of the raw MSW from which they are made. This will not however necessarily preclude international trade in RDF pellets, as RDF pellets manufactured with close attention to quality are far superior to raw MSW in fuel applications. That a country should purchase high-quality RDF pellets whilst disposing of its own MSW by simple incineration or landfill is no more anomalous than transport of raw waste between Canada and the US for landfill disposal which, as we have already noted, is currently taking place.












0 comments:
Post a Comment
Please wait for approval of your comment .......